2019-nCoV Nucleocapsid ELISA Kit

- 1
- Product Details
- How to order
- Instructions
This assay recognizes natural and recombinant nucleocapsid protein in bronchoalveolar lavage fluid, nasopharyngeal swab samples or recombinant samples.
The kit is stored at the recommended temperature for 6 months, and the signal intensity decreases by less than 10%
OTHER SUPPLIES REQUIRED
• Microplate reader capable of measuring absorbance at 450 nm, with the correction wavelength set at 540 nm or 570 nm.
• Pipettes and pipette tips.
• Deionized or distilled water.
• 500 mL graduated cylinder.
• Squirt bottle, manifold dispenser, or automated microplate washer.
• Test tubes for dilution of standards.
REAGENT PREPARATION
Bring all reagents to room temperature before use.
1. 20-fold Wash Buffer Concentrate: if there is crystal precipitation in the Wash Buffer Concentrate, warm to room temperature and mix gently until the crystals have completely dissolved. Add 25 mL of Wash Buffer Concentrate to 475 mL of deionized or distilled water to prepare 500 mL of Wash Buffer.
2. Reconstitute the Standard with 2mL Standard/Sample Dilution Buffer, The undiluted nucleocapsid protein Standard (80 ng/mL) serves as the high standard. The multiple proportion dilution of the standard was selected. The concentration of the 7 standard sample were 80ng/mL, 40ng/mL, 20ng/mL, 10ng/mL, 5ng/mL, 2.5ng/mL, 1.25ng/mL respectively. The appropriate calibrator diluent serves as the Standard/Sample Dilution Buffer (0 pg/mL).
3. Detection A (biotin labeled) shake and mix before used. Centrifuge instantaneously with palm centrifuge to make the liquid at the bottom of the tube. Dilute the Detection A 1:100 to the working concentration with Reagent Dilution Buffer.
4. Detection B (HRP labeled) shake and mix before used, Centrifuge instantaneously with palm centrifuge to make the liquid at the bottom of the tube. Dilute the Detection B 1:100 to the working concentration with Reagent Dilution Buffer.
5. Color Reagent TMB: Protect from light, Add 100μL of Color Reagent TMB to each well. Incubate for 5-10 minutes at room temperature.
6. Stop solution: 50μL/well after color development.
SAMPLE COLLECTION & STORAGE
The sample collection and storage conditions listed below are intended as general guidelines. Sample stability has not been evaluated.
Cell Culture Supernates - Remove particulates by centrifugation and assay immediately or aliquot and store samples at ≤ -20 ℃. Avoid repeated freeze-thaw cycles.
ASSAY PROCEDURE
Bring all reagents and samples to room temperature before use. It is recommended that all standards, controls, and samples be assayed in duplicate.
1. Prepare all reagents and working standards as directed in the previous sections.
2. Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
3. Add 100μL of standard, control, or samples per well. Cover with the adhesive strip provided. Incubate for 1 hour at 37℃.
4. Aspirate each well and wash, repeating the process three times. Wash by filling each well with Wash Buffer (300 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential to good performance. After the last wash, remove any remaining Wash Buffer by aspirating or decanting. Invert the plate and blot it against clean paper towels.
5. Add 100μL of Detection A (biotin labeled) antibody to each well. Cover with a new adhesive strip. Incubate for 0.5 hour at 37℃.
6. Repeat the aspiration/wash as in step 4.
7. Add 100μL of Detection B (HRP labeled) to each well. Cover with a new adhesive strip. Incubate for 0.5 hour at 37℃.
8. Repeat the aspiration/wash as in step 4.
9. Add 100μL of Color Reagent TMB to each well. Incubate for 5-10 minutes at 37℃. Protect from light.
10. Add 50μL of Stop Solution to each well. The color in the wells should change from blue to yellow. If the color in the wells is green or the color change does not appear uniform, gently tap the plate to ensure thorough mixing.
11. Determine the optical density of each well within 10 minutes, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
CALCULATION OF RESULTS
Average the duplicate readings for each standard, control, and sample and subtract the average zero standard optical density (O.D.).
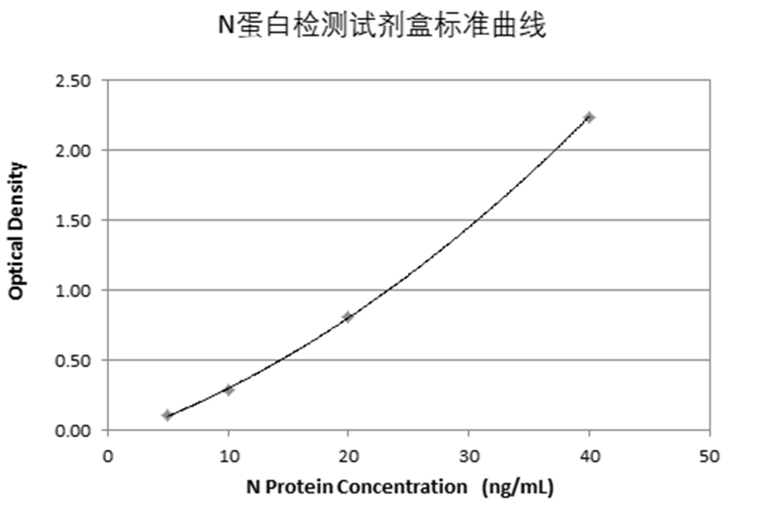
Create a standard curve by reducing the data using computer software capable. Construct a standard curve by plotting the mean absorbance for each standard on the Y-axis against the concentration on the X-axis and draw a best fit curve through the points on the graph. The data may be linearized by plotting the log of the nucleocapsid protein concentrations versus the log of the O.D. and the best fit line can be determined by regression analysis. This procedure will produce an adequate but less precise fit of the data.
If samples have been diluted, the concentration read from the standard curve must be multiplied by the dilution factor.
LINEARITY
1.25ng/mL—80ng/mL
SENSITIVITY
The minimum detectable dose (MDD) of nucleocapsid protein is typically less than 1ng/mL.
The MDD was determined by adding two standard deviations to the mean O.D. value of twenty
zero standard replicates and calculating the corresponding concentration.
Precision
Intra-Assay Precision (Precision within an assay): <12%
Three samples of known concentration were tested twenty times on one plate to assess
intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty separate assays to assess
inter-assay precision.
Stability
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 10%.
MATERIALS PROVIDED & STORAGE CONDITIONS(Provided this is within the expiration date of the kit.)
|
PART |
Format |
Description |
STORAGE CONDITIONS |
|
Capture Plate |
1 plate |
96 well polystyrene microplate (12 strips of 8 wells) coated with a rabbit polyclonal antibody specific for nucleocapsid protein. |
Store in sealed at - 20℃. |
|
Standard |
1 bottle |
160ng/bottle recombinant nucleocapsid protein in a buffered protein base with preservatives, lyophilized. Dissolute in 2ml deionized water before used. Final concentration was 80ng/mL. |
Store at - 20℃. |
|
Detection A |
1 vial |
120μL/vial biotin labeled anti- Nucleocapsid protein monoclonal antibody (including preservative) , 1:100 diluted by dilution buffer before used. |
Store at - 20℃. |
|
Detection B |
1 vial |
120μL/vial streptavidin conjugated to HRP with preservatives, 1:100 diluted by dilution buffer before used. |
Store at - 20℃. |
|
Standard/Sample dilution Buffer |
1 bottle |
25 ml/bottle diluent (including preservative) was used to dilute the standard or sample. |
Store at 4℃. |
|
Reagent Dilution Buffer |
1 bottle |
25 ml/bottle diluent (including preservative) was used to dilute the Detection A and B. |
Store at 4℃. |
|
Wash Buffer Concentrate |
1 bottle |
25 mL/bottle of a 20-fold concentrated solution of buffered surfactant with preservative. 1:20 diluted by deionized water before used. |
Store at 4℃. |
|
Color Reagent TMB |
1 bottle |
12 mL/ bottle of TMB(tetramethylbenzidine) . |
Store at 4℃. |
|
Stop Solution |
1 bottle |
6 mL/ bottle. |
Store at 4℃. |
|
Plate Sealer |
4 strips |
Adhesive strips. |
RT. |
SPECIFICITY
This assay recognizes natural and recombinant nucleocapsid protein in bronchoalveolar lavage fluid, nasopharyngeal swab samples or recombinant samples.
Guarantee and Disclaimer:
After receiving the product, if finds that the product is mismatched, damaged or missing components, please keep the original package and submit the objection to the company by mail within seven working days. Failure to file an objection within the time limit is considered qualified.
When the buyer keeps the product, it should be kept in accordance with the storage conditions shown in the product label and the manual. If the product quality is caused by improper storage, it will not be guaranteed.
When the buyer tests the product, they should be submitted by the beginning of use when found the quality issue, rather than when the product was used or used up, in order to prepare our products for recycling and confirm the quality of the products. If it is the quality problem, our company is responsible for exchange or return. In the event of a claim, our company will compensate the discretion within the scope of the product price limit and will not accept any part of the value of the product itself. Since the date of receipt, the product has not been reflected for more than three months and should not be returned.
The products provided by our company are for research use only and should not be used for clinical diagnosis or treatment. If a unit or individual changes the use of our products without authorization, we will not bear any responsibility.
Copyright © 2024 Lawyer Theme All Rights Reserved


