TUNEL Apoptosis Detection Kit(Fluorescein-12)

- 1
- Product Details
- How to order
- Instructions
(1) Phosphate buffer PBS;
(2) 4% paraformaldehyde solution dissolved in PBS, pH 7;
(3) 1% Triton X-100 dissolved in PBS;
(4) DAPI (2 μ g / ml) or PI (1 μ g / ml) should be prepared for nuclear staining;
(5) DNase I buffer and DNase I should be prepared if positive control group is needed;
(6) For your safety and health, please wear lab clothes and disposable gloves.
2. Sample pretreatment
2.1 cell samples
(1) The cell slide or smear was washed with PBS for 5 minutes, then fixed with 4% paraformaldehyde solution dissolved in PBS at room temperature for 30 minutes;
(2) Wash with PBS for 5 minutes;
(3) The solution of PBS containing 0.1% Triton X-100 was used, and the solution was soaked at 37 ℃ for 10-15 minutes;
(4) PBS wash 3 times, 5 minutes each time;
Note: the cell smear should be removed properly.
2.2 tissue section
2.2.1 paraffin section
(1) The paraffin sections were dewaxed in xylene for 10-20 minutes at room temperature, and then dewaxed with fresh xylene for 10-20 minutes, repeated 1-2 times;
(2) At room temperature, the slices were soaked in 100% ethanol for 5 minutes and repeated twice;
(3) The slices were soaked in gradient ethanol (95%, 80%, 70%) for 5 minutes at room temperature;
(3) Soak and wash in PBS for 5 minutes;
Note: during the experiment, do not let the sample dry, and keep the treated sample wet in the wet box.
(4) Preparation of proteinase K working solution: 1:9 volume ratio, PBS as diluent, to the final concentration of 0.2 μ g / μ L;
(5) Each sample was dripped with 100 μ l proteinase K working solution to cover the sample, and the wet box was permeable at 37 ℃ for 15-20 minutes;
Note: proteinase K can help tissues and cells to penetrate the dye. Too long incubation time will cause tissues or cells to fall off from the slices. Too short incubation time will cause insufficient permeability treatment, which will affect the efficiency of subsequent labeling. In order to get better results, we can optimize the incubation time of proteinase K.
(6) PBS washed 3 times, 5 minutes each time; after treatment, the samples were placed in the wet box to keep the samples moist.
Note: protease K must be washed clean in this step, otherwise it will seriously interfere with the subsequent labeling reaction.
2.2.2 frozen section
(1) The slides were immersed in 4% paraformaldehyde solution dissolved in PBS and incubated at room temperature for 30 minutes.
(2) After the slide is taken out from the fixed solution, it is put in the fume hood to dry naturally;
(3) Soak and wash the glass slide in pure water or PBS for 3-5 minutes;
(4) Each sample was dripped with 100 μ l proteinase K working solution to cover the sample, and the wet box was permeable at 37 ℃ for 15-20 minutes;
(5) PBS washed 3 times, 5 minutes each time; after treatment, the samples were placed in the wet box to keep the samples moist.
3. Treatment of positive and negative samples (optional)
(1) Positive control group: 10 U / ml DNase I was added;
(2) Negative control group: equal volume of 1 × DNase I buffer was added;
(3) All groups were added with 1 × DNase I buffer and completely covered the sample surface, and incubated in 37 ℃ wet box for 30 minutes.
(4) Remove the excess liquid and wash the slide thoroughly with deionized water for 3-4 times.
4. Staining mark
Configuration table of TUNEL test solution:
Note: TUNEL test solution should be prepared and used now to avoid repeated freezing and thawing.
(1) Dilute 10x equilibrium buffer with ddH2O in the ratio of 1:9 to 1x equilibrium buffer for standby;
(2) Prepare TUNEL test solution according to the table proportion, add appropriate amount of TUNEL test solution to each group of slices to make it completely covered (for smear, slice or 96 well plate, 48 well plate, 24 well plate and 12 well plate, generally 50 μ l TUNEL test solution can be enough for the sample size of about 2.5 * 2.5cm), note that the slices should not be dry and should be kept away from light;
(3) The slides were incubated in a 37 ℃ wet box for 1-2 hours without light;
(4) Immediately moisten with PBS solution for 3-4 times, 5 minutes each time;
(5) Immerse the glass slide in the dyeing vat of PI solution (1 μ g / ml) or DAPI solution (2 μ g / ml) in the dark environment and leave it at room temperature for 8 minutes (optional);
(6) The slides were washed with PBS for 5 minutes three times;
(7) Filter paper was used to absorb the excess liquid of glass slide, and anti fluorescence quenching sealing agent was used to seal the slide.
Note: before sealing, add 100 μ l PBS containing anti fluorescence quenching agent and 20% glycerin to the sample area to keep the sample moist.
5. Observation and detection
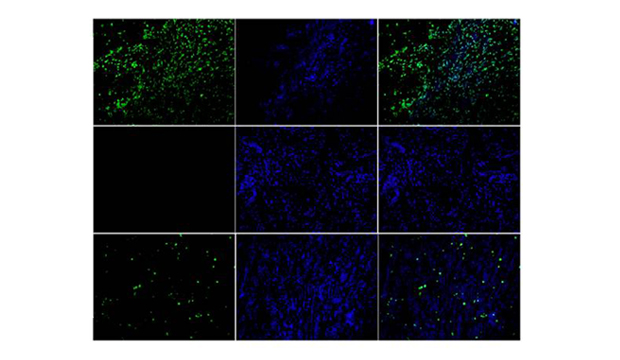
Observe under fluorescence microscope, avoid light. Pi / DAPI could dye the apoptotic / non apoptotic cells red / blue. Fitc-12-dutp was only incorporated into the apoptotic nucleus and localized green fluorescence.
6. Suspension cells were detected by flow cytometry
(1) The 3.0-5.0 × 106 cells were washed with PBS twice, centrifuged at 4 ℃ and 1500 rpm for 10 minutes each time;
(2) Cells were resuspended with 0.5 ml PBS;
(3) The cells were fixed on 1 ml polyformaldehyde solution for 30 minutes;
(4) After centrifugation at 4 ℃ and 1500 rpm for 5 minutes, the supernatant was removed;
(5) The cells were resuspended with 1 ml PBS and centrifuged at 4 ℃ and 1500 rpm for 5 minutes. The supernatant was removed and resuspended with 0.5 ml PBS;
(6) 1 ml PBS solution containing 0.1% Triton X-100 or 0.2 μ g / μ L protein K solution was used to permeate the cells, and the cells were placed at room temperature for 5 minutes;
(7) After centrifugation at 1500 rpm for 5 minutes, the supernatant was discarded and the cells were resuspended with 1 ml PBS;
(8) The cells were transferred to a new 1.5 ml micro centrifuge tube and centrifuged at 1500 rpm for 5 minutes to remove the supernatant;
(9) The cells were resuspended with 80 μ L 1 x equilibrium buffer and incubated at room temperature for 30 minutes;
(10) Centrifugation at 1500 rpm for 10 minutes to remove the supernatant;
(11) The cell precipitates were resuspended in 50 μ l TUNEL detection solution (refer to the configuration table of TUNEL detection solution), incubated at 37 ℃ for 1-2 h in dark, and resuspended with micro pipette every 15 min;
(12) After the reaction, 1 ml of 20 mm EDTA was added to terminate the reaction, and the micro pipette was used to mix well;
(13) After centrifugation at 1500 rpm for 10 minutes, the supernatant was removed and the cell precipitate was resuspended with 0.5 ml PI solution (1 μ g / ml) and incubated in dark for 30 minutes;
(14) The cells were analyzed by flow cytometry. The green fluorescence of fluorescein-12-dutp in 495-520 nm and the red fluorescence of PI in > 620 nm were measured.
7. Common problems and precautions
(1) Fluorescent high background
1. It is necessary to add FITC in the whole process;
2. In the cells with high speed of division or proliferation, sometimes the nuclear DNA breaks;
3. TUNEL reaction was too strong. The rtdt enzyme can be diluted 2-5 times with the rtdt enzyme diluent provided by the kit before operation. The diluted rtdt enzyme should be used up on the same day;
4. Mycoplasma contamination. Mycoplasma contamination was detected by Mycoplasma staining kit;
(2) The fluorescence signal is weak
1. The concentration and incubation time of the Permeabilizer can be adjusted if the permeability of Triton X-100 or protease K is not enough;
2. Fluorescence quenching. The fluorescent agent should be stored and used away from light;
3. Due to the weak adherence of apoptotic cells, we should operate gently to avoid cell loss;
(3) There were nonspecific markers
1. The activity of DNase in some cells and tissues is relatively high, which easily leads to non-specific markers. The solution is to fix the cells or tissues immediately after taking them to prevent enzyme activity;
2. The contamination of DNA enzyme may be mixed in the process of operation.
The TUNEL cell apoptosis Kit (fluorescein-12) produced by our company is a high sensitivity, which can detect apoptosis at the level of single cell and detect early apoptosis at the same time. It is fast and simple. After staining and washing the fixed cells or tissues, it can be detected under the fluorescence microscope or flow cytometry. The whole experimental operation only takes 1-2 hours. In addition, the kit can be used in a wide range of applications, not only for the detection of apoptosis in frozen sections and paraffin sections, but also for the detection of apoptosis in adherent or suspended cells.
Guarantee and Disclaimer:
After receiving the product, if finds that the product is mismatched, damaged or missing components, please keep the original package and submit the objection to the company by mail within seven working days. Failure to file an objection within the time limit is considered qualified.
When the buyer keeps the product, it should be kept in accordance with the storage conditions shown in the product label and the manual. If the product quality is caused by improper storage, it will not be guaranteed.
When the buyer tests the product, they should be submitted by the beginning of use when found the quality issue, rather than when the product was used or used up, in order to prepare our products for recycling and confirm the quality of the products. If it is the quality problem, our company is responsible for exchange or return. In the event of a claim, our company will compensate the discretion within the scope of the product price limit and will not accept any part of the value of the product itself. Since the date of receipt, the product has not been reflected for more than three months and should not be returned.
The products provided by our company are for research use only and should not be used for clinical diagnosis or treatment. If a unit or individual changes the use of our products without authorization, we will not bear any responsibility.
Copyright © 2024 Lawyer Theme All Rights Reserved


