Recombinant Human CD52 protein ,C- Fc Tag
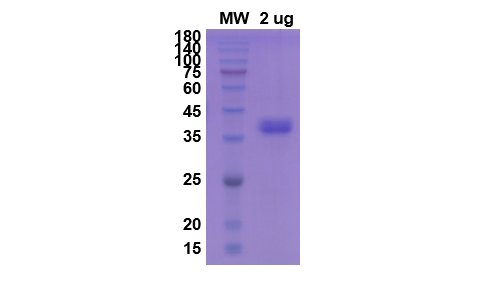

- 1
- Product Details
- Frontier progress
- How to order
- Instructions
Store at 2 to 8 °C for one week .
Store at -20 to -80 °C for twelve months from the date of receipt.
Guarantee and Disclaimer:
After receiving the product, if finds that the product is mismatched, damaged or missing components, please keep the original package and submit the objection to the company by mail within seven working days. Failure to file an objection within the time limit is considered qualified.
When the buyer keeps the product, it should be kept in accordance with the storage conditions shown in the product label and the manual. If the product quality is caused by improper storage, it will not be guaranteed.
When the buyer tests the product, they should be submitted by the beginning of use when found the quality issue, rather than when the product was used or used up, in order to prepare our products for recycling and confirm the quality of the products. If it is the quality problem, our company is responsible for exchange or return. In the event of a claim, our company will compensate the discretion within the scope of the product price limit and will not accept any part of the value of the product itself. Since the date of receipt, the product has not been reflected for more than three months and should not be returned.
The products provided by our company are for research use only and should not be used for clinical diagnosis or treatment. If a unit or individual changes the use of our products without authorization, we will not bear any responsibility.
Copyright © 2024 Lawyer Theme All Rights Reserved


